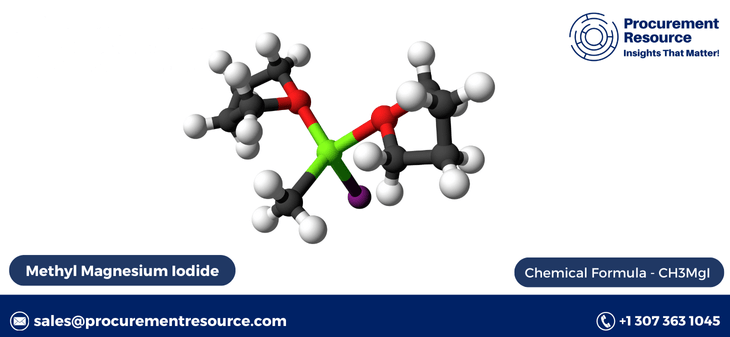
Methyl magnesium iodide, a Grignard reagent, is a key compound used in organic synthesis, particularly for the formation of carbon-carbon bonds. It plays a crucial role in numerous chemical reactions, such as the addition of alkyl or aryl groups to carbonyl compounds, which are fundamental steps in the production of pharmaceuticals, agrochemicals, and fine chemicals. The production process of methyl magnesium iodide requires precision, expertise, and a thorough understanding of the reagents and conditions involved. This blog provides an in-depth exploration of the methyl magnesium iodide production process, its significance, and how resources like Procurement Resource can assist businesses in optimizing production costs and understanding the market landscape.
Why Methyl Magnesium Iodide Production Process Matters
The importance of methyl magnesium iodide in chemical synthesis cannot be overstated. It serves as a cornerstone for numerous organic reactions, such as nucleophilic additions to aldehydes, ketones, and esters, enabling the formation of complex molecular structures. This compound is indispensable in the pharmaceutical industry, where it is used to synthesize active pharmaceutical ingredients (APIs), and in the agrochemical sector for creating various plant protection products.
Request a Free Sample – https://www.procurementresource.com/production-cost-report-store/methyl-magnesium-iodide/request-sample
Understanding the production process of methyl magnesium iodide is essential not only for chemical manufacturers but also for companies that rely on this compound as a precursor or reagent. An efficient and cost-effective production process can significantly impact the pricing, availability, and quality of the final product. For businesses involved in the procurement of raw materials, having detailed insights into the production process helps in making informed decisions regarding supply chain management, vendor selection, and cost optimization.
Read Full Report – https://www.procurementresource.com/production-cost-report-store/methyl-magnesium-iodide
Moreover, regulatory requirements around the handling and production of Grignard reagents add another layer of complexity to the process. Ensuring compliance with environmental, health, and safety standards is critical to avoid potential liabilities and operational disruptions. This is where comprehensive production process reports, like those offered by Procurement Resource, can be invaluable.
The Power of Procurement Resource Methyl Magnesium Iodide Production Process Reports
Procurement Resource provides detailed production process reports that offer valuable insights into the entire lifecycle of methyl magnesium iodide production. These reports are designed to empower procurement managers, decision-makers, and business leaders by providing them with an understanding of the technical and economic aspects of the production process. Here’s what makes these reports an essential tool for businesses involved in the chemical industry:
Ask an Analyst – https://www.procurementresource.com/production-cost-report-store/methyl-magnesium-iodide/ask-an-analyst
- Detailed Breakdown of the Production Process:
Procurement Resource reports cover every step of the methyl magnesium iodide production process. This includes the preparation of raw materials, reaction conditions, purification methods, and storage requirements. By understanding these steps, companies can identify potential areas for optimization, such as reducing waste, improving yields, or adopting more energy-efficient methods. - Cost Analysis:
One of the most critical aspects of any production process is cost. The report provides a comprehensive analysis of the costs associated with the production of methyl magnesium iodide. This includes the cost of raw materials (such as methyl iodide and magnesium), labor, energy, equipment, and overheads. Having access to this data allows businesses to benchmark their own production costs against industry standards and identify areas for cost reduction. - Market Dynamics:
The production process is closely tied to market conditions, such as the availability and pricing of raw materials. Procurement Resource’s reports include an analysis of market trends, supply chain dynamics, and potential risks that could affect the availability or cost of methyl magnesium iodide. This helps businesses prepare for fluctuations in the market and develop strategies to mitigate potential risks. - Sustainability and Compliance:
Environmental sustainability and regulatory compliance are becoming increasingly important in chemical production. Procurement Resource reports provide insights into the environmental impact of the methyl magnesium iodide production process, including waste management and energy consumption. The reports also highlight relevant regulations and compliance requirements, ensuring that businesses remain in line with legal and environmental standards. - Benchmarking and Best Practices:
By comparing different production methods, Procurement Resource reports enable companies to benchmark their processes against industry best practices. This can lead to improved operational efficiency, reduced costs, and higher product quality. The reports also offer recommendations for process improvements based on the latest technological advancements and industry trends.
How Procurement Resource Can Help You
Whether you are a procurement professional, a production manager, or a business leader, having access to reliable and detailed information about the methyl magnesium iodide production process can be a game-changer. Procurement Resource offers a wealth of knowledge that can help your business make informed decisions, optimize production processes, and stay ahead of the competition.
Here’s how Procurement Resource can specifically help your business:
- Cost Optimization: By providing detailed cost analyses, Procurement Resource helps you identify areas where you can reduce production costs, improve efficiency, and increase profitability. Whether it’s optimizing raw material procurement, reducing energy consumption, or streamlining labor costs, the insights provided by these reports are invaluable.
- Supply Chain Management: With an in-depth understanding of market dynamics, including the availability and pricing of raw materials, Procurement Resource helps you manage your supply chain more effectively. This includes identifying reliable suppliers, negotiating better terms, and mitigating risks related to raw material shortages or price fluctuations.
- Regulatory Compliance: Ensuring that your production process complies with environmental and safety regulations is essential for avoiding costly fines and reputational damage. Procurement Resource reports provide a thorough overview of relevant regulations and best practices for handling hazardous chemicals like methyl magnesium iodide.
- Market Intelligence: Understanding the broader market trends can help you make strategic decisions regarding production capacity, product pricing, and market entry. Procurement Resource reports include market forecasts, competitor analyses, and insights into emerging technologies that can give your business a competitive edge.
- Risk Management: By identifying potential risks in the production process, such as supply chain disruptions or regulatory changes, Procurement Resource helps you develop contingency plans and strategies for risk mitigation.
Request Your Free Sample Report Today
If you are looking to gain a competitive advantage in the production and procurement of methyl magnesium iodide, now is the time to act. Procurement Resource offers a free sample report that provides a sneak peek into the wealth of information available in their comprehensive production process reports.
Request Your Free Sample Report – https://www.procurementresource.com/production-cost-report-store/methyl-magnesium-iodide/request-sample
By requesting your free sample report, you will gain access to key insights that can help your business optimize its operations, reduce costs, and improve its supply chain management. Don’t miss out on this opportunity to enhance your understanding of the methyl magnesium iodide production process and take your business to the next level.
The production of methyl magnesium iodide is a complex process with numerous variables that can affect cost, efficiency, and product quality. Having access to detailed production process reports, like those provided by Procurement Resource, can provide businesses with the insights they need to optimize their operations and stay competitive in the market. From cost analysis to regulatory compliance and market intelligence, these reports offer invaluable information that can drive business success. Request your free sample report today and start unlocking the potential of the methyl magnesium iodide production process.
Contact Us:
Company Name: Procurement Resource
Contact Person: Endru Smith
Email: sales@procurementresource.com
Toll-Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537171117 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA