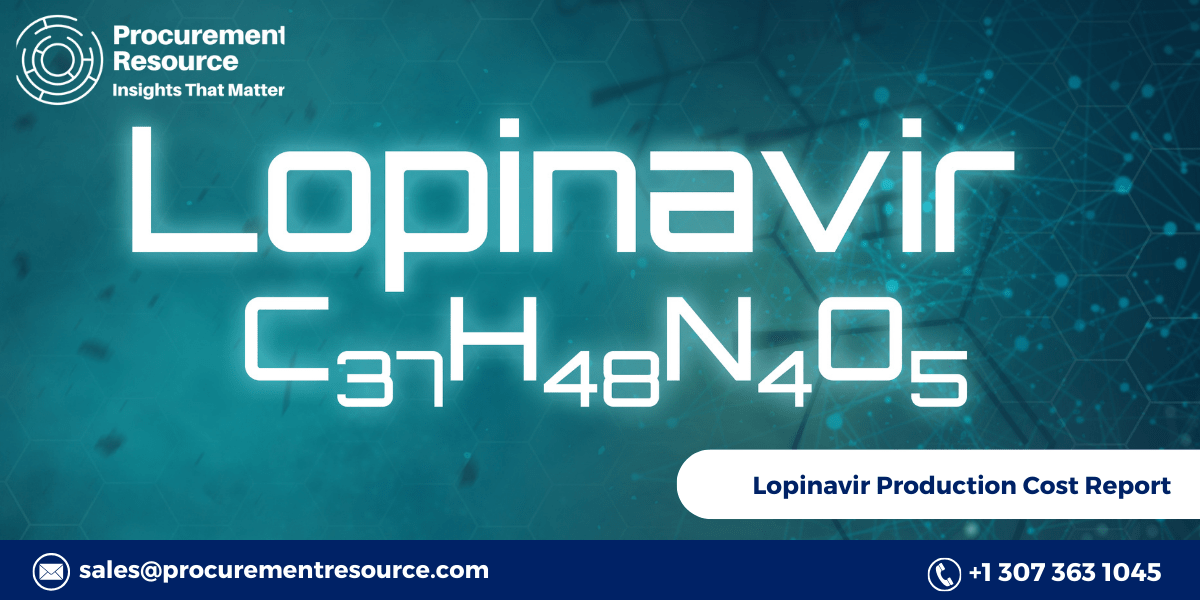
Lopinavir, a crucial antiretroviral medication used in the treatment of HIV/AIDS, has been at the forefront of global healthcare discussions. The production cost of lopinavir is a significant factor influencing its availability and affordability, especially in low- and middle-income countries where the burden of HIV/AIDS is most prevalent. Understanding the production cost of Lopinavir involves examining the entire manufacturing process, including the raw materials required, the production techniques employed, and the latest advancements in the field. This report provides an in-depth analysis of Lopinavir production costs, offering insights into the factors that drive these costs and the implications for global healthcare.
Production Process
The production of Lopinavir involves a multi-step chemical synthesis process. The active pharmaceutical ingredient (API) of Lopinavir is synthesized through a series of chemical reactions that require precision and high-quality raw materials. The process typically begins with the synthesis of key intermediates, which are then combined through catalytic processes to form Lopinavir.
Request For Sample: https://www.procurementresource.com/production-cost-report-store/lopinavir/request-sample
One of the critical steps in Lopinavir production is the protection and deprotection of functional groups, ensuring that the final product has the desired pharmacological properties. The synthesis process also involves multiple purification stages, including crystallization and filtration, to achieve the required purity levels for pharmaceutical use. The entire production process is conducted under stringent quality control measures to ensure that the final product meets international standards for safety and efficacy.
The complexity of the production process directly impacts the cost of Lopinavir. Each step requires specific reagents, catalysts, and conditions that contribute to the overall production cost. Additionally, the need for specialized equipment and skilled labor further adds to the cost of production.
Manufacturing Report and Process
The manufacturing of Lopinavir is typically carried out in a pharmaceutical-grade facility equipped with state-of-the-art technology. The manufacturing process involves both chemical synthesis and formulation stages, where the active pharmaceutical ingredient is combined with other excipients to create the final dosage form, such as tablets or capsules.
The manufacturing process begins with the large-scale synthesis of Lopinavir in reactors designed to handle the specific chemical reactions involved. The reactors are equipped with advanced monitoring systems to ensure that the reaction conditions, such as temperature and pressure, are maintained within the optimal range. Once the synthesis is complete, the crude product undergoes several purification steps to remove impurities and by-products.
The purified Lopinavir is then formulated into the final dosage form. This involves mixing the API with excipients, which are inactive ingredients that help in the drug’s delivery and stability. The mixture is then compressed into tablets or filled into capsules using high-speed machines. The final product is subjected to rigorous quality testing, including dissolution, potency, and stability tests, to ensure it meets all regulatory requirements.
The manufacturing report also includes a detailed analysis of the production yields at each stage, highlighting areas where process optimization could reduce costs. By identifying steps in the manufacturing process that could be streamlined or improved, pharmaceutical companies can potentially lower the overall production cost of Lopinavir.
Raw Material Costs
The cost of raw materials is a major component of the overall production cost of Lopinavir. The synthesis of Lopinavir requires several chemical reagents, solvents, and catalysts, each contributing to the cost structure. The prices of these raw materials can fluctuate based on market conditions, availability, and the scale of production.
Key raw materials used in Lopinavir production include organic chemicals such as amines, acids, and alcohols. The cost of these chemicals is influenced by factors such as global supply chains, the cost of crude oil (a base for many organic chemicals), and geopolitical factors that may affect the availability of raw materials. Additionally, the procurement of high-purity reagents required for pharmaceutical-grade production can be more expensive, further driving up the production costs.
Pharmaceutical companies often seek to negotiate bulk purchasing agreements with suppliers to reduce the cost of raw materials. However, fluctuations in the market can still lead to variability in production costs. The cost of catalysts and solvents, which are typically used in large quantities during the synthesis process, also plays a significant role in the overall cost structure.
Understanding the cost dynamics of raw materials is crucial for pharmaceutical companies, as it allows them to better predict production costs and set pricing strategies for the final product. By optimizing the use of raw materials and exploring alternative suppliers, companies can reduce the impact of raw material costs on the overall production expense.
Latest News
Recent developments in the pharmaceutical industry have brought attention to the production costs of essential medications like Lopinavir. Advances in chemical synthesis techniques and the adoption of green chemistry practices are among the latest trends that could potentially reduce production costs.
Green chemistry, which focuses on minimizing waste and using environmentally friendly processes, is gaining traction in the pharmaceutical industry. For Lopinavir production, this could mean the development of more efficient synthesis routes that reduce the need for expensive and hazardous chemicals. Additionally, the use of biocatalysts, which are enzymes that can catalyze chemical reactions under mild conditions, is being explored as a way to lower production costs and improve sustainability.
Another significant development is the increased focus on local manufacturing of essential medicines in low- and middle-income countries. Governments and international organizations are investing in the establishment of pharmaceutical manufacturing facilities in these regions to reduce dependency on imports and make essential medicines like Lopinavir more affordable and accessible. This shift towards local production could also lead to cost reductions by eliminating transportation and import duties.
In the context of the COVID-19 pandemic, Lopinavir gained attention as a potential treatment option, leading to a surge in demand. Although subsequent studies did not support its efficacy against COVID-19, the increased focus on Lopinavir production highlighted the need for efficient and cost-effective manufacturing processes. Pharmaceutical companies are now more than ever looking to streamline production and reduce costs to ensure that essential medicines remain accessible to those in need.
Contact Us:
Company Name: Procurement Resource
Contact Person: Endru Smith
Email: sales@procurementresource.com
Toll-Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: https://www.procurementresource.com/